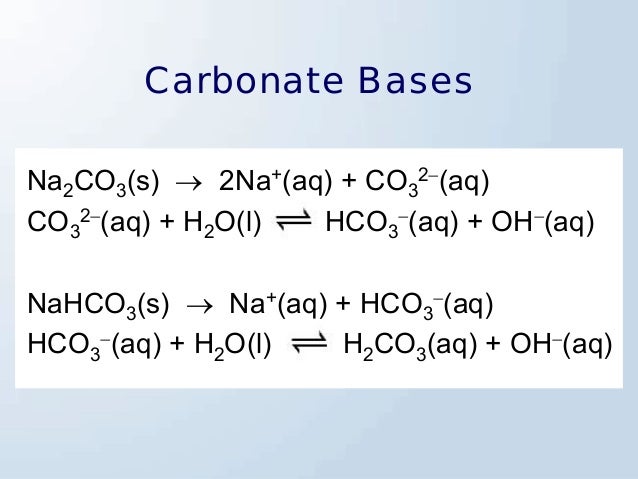
Is Na2co3 Basic Or Acidic . Is na2co3 an acid or base? Is naclo4 an acid or base?
PPT Chapter 18 Equilibria Involving Acids & bases PowerPoint Presentation ID197628 from www.slideserve.com
Is naclo4 an acid or base? A salt in chemistry is formed by the reaction of an acid and a base. Read, more elaboration about it is given here.
PPT Chapter 18 Equilibria Involving Acids & bases PowerPoint Presentation ID197628
Is na2co3 acidic basic or neutral? Its chemical formula is written as alcl3. Now depending on the strength of the respective acid and base from which the salt is derived, it may be categorised as an acidic, basic or a neutral salt. Which solution is basic in nature?
Source: www.slideserve.com
Is na2co3 acidic basic or neutral? Naclo4 is not an acid at all. Na2co3 is neither an acid nor a base. Why aqueous solution is basic in nature? That was my logic in answering this problem.
Source: www.slideserve.com
One way a person can view this is as follows: Sodium chloride, which is obtained by neutralization of hydrochloric acid and sodium hydroxide, is a neutral salt. If you consider h2so4 to be a strong acid that donates both protons fully then na2so4 will be neutral and nahso4. Also asked, why is nacl not an acid? Should na (+) attempt.
Source: www.slideshare.net
Page contents show why sodium carbonate (na2co3) is basic salt in nature? A salt in chemistry is formed by the reaction of an acid and a base. The ph value of the aqueous solution of sodium carbonate is greater than 7. Na2co3 is neither an acid nor a base. So we see that it gives a naoh, a strong base.
Source: www.youtube.com
A salt in chemistry is formed by the reaction of an acid and a base. Na2co3 is neither an acid nor a base. Na2co3 is neither an acid nor a base. It is generally referred to as washing soda and is used as a food additive, in cleaning products, glass processing, and more. Is na2co3 acidic basic or neutral?
Source: www.slideserve.com
So, the more na+ is hydrolysed more oh− are produced, hence aqueous solution of na2co3 is basic in nature. First of all, solid naoh absorbs water from the air, so accurately weighing a sample during the preparation of a solution is impossible. Is this the logic of the correct answer is following? Read, more elaboration about it is given here..
Source: www.slideshare.net
It can be an acidic salt because all its hydrogen ions are not replaced.at the same time,it can be a basic salt beacuse it will further react with an acid to produce a normal salt and it is possible only for a. When dissolved in water this compound actually produces a neutral solution. That was my logic in answering this.
Source: www.slideshare.net
Is na2co3 acidic basic or neutral? Is nano3 an acid or base? Is nano3 a base or an acid? So, the more na+ is hydrolysed more oh− are produced, hence aqueous solution of na2co3 is basic in nature. Is naclo4 an acid or base?